Better Health Through Better Bacteria
The Human Microbiome Project (HMP) is a fascinating program being funded by the National Institute of Health (NIH) Common Fund. The human microbiome has been defined as: “the ecological community of commensal, symbiotic, and pathogenic microorganisms that literally share our body space”. The number of bacteria living within the body of the average healthy adult human are estimated to outnumber human cells 10 to 1. What is not well understood today is how the proportions and/or the presence of bacteria and/or fungi with a specific genetic constitution might be involved in making people sick.
What is the Human Microbiome Project?
As part of the NIH Common Fund’s Roadmap for Medical Research, the HMP, launched in 2008, a is a $157 million, five-year effort that will support a series of studies to better understand the role of the microbiome in human health. The aim of the HMP is to “characterize microbial communities found at multiple human body sites and to look for correlations between changes in the microbiome and human health”.
How Varied are the Bacteria?
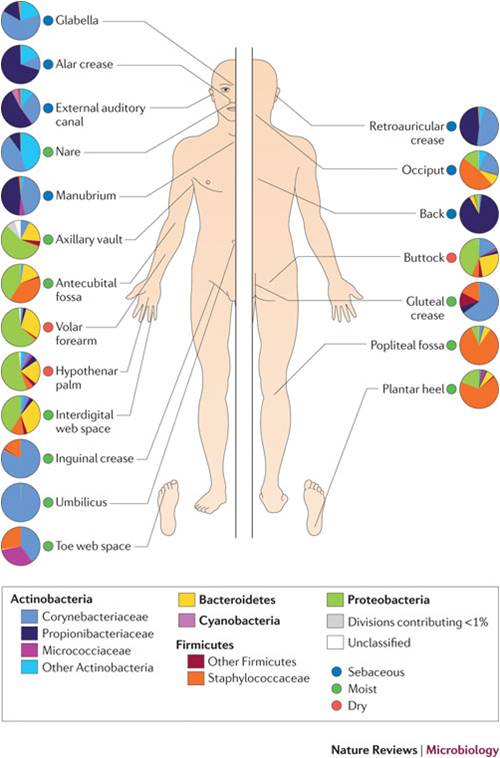
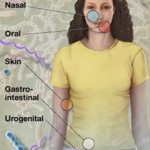
Grice and Segre’s work provides insight into the variability of organisms populating various areas of the epidermis. As seen in the figure, wide variability in distribution by body site exists. Current and future work will further investigate how the genetic lineage may vary between these bacterial populations and if there exists a consistent disease-related genetic pattern. This type of investigation will hopefully lead to therapies for those conditions related to particular genetically constituted bacteria.
Areas of Investigation
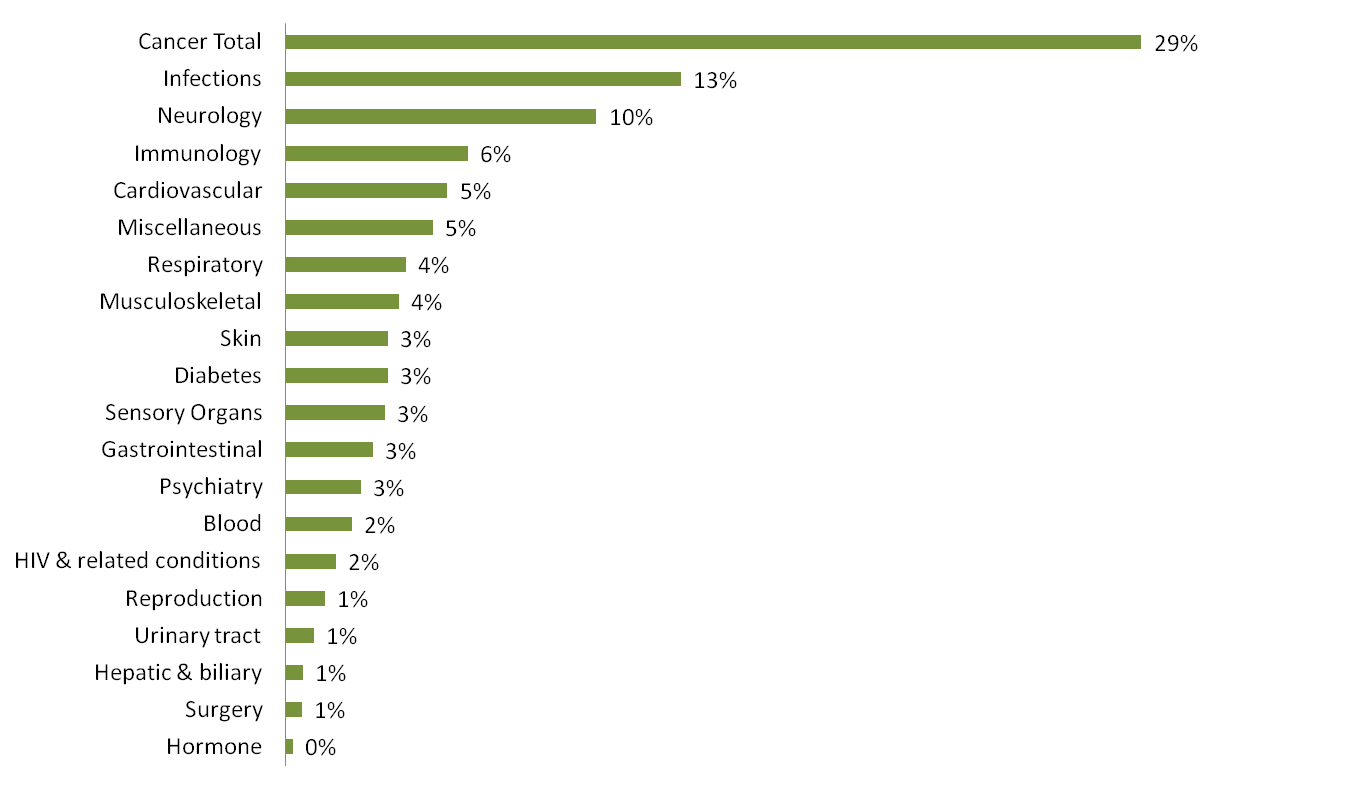
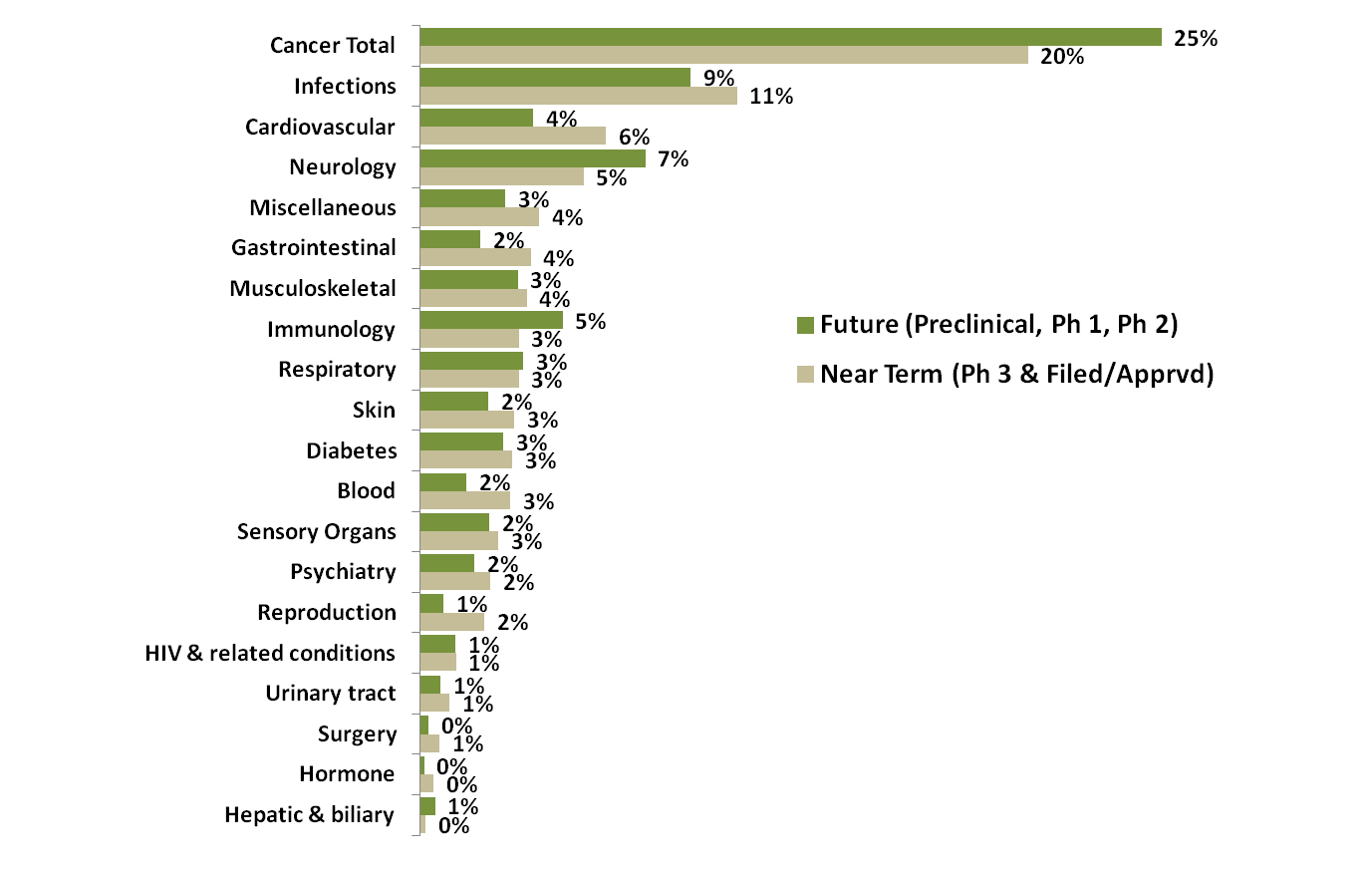
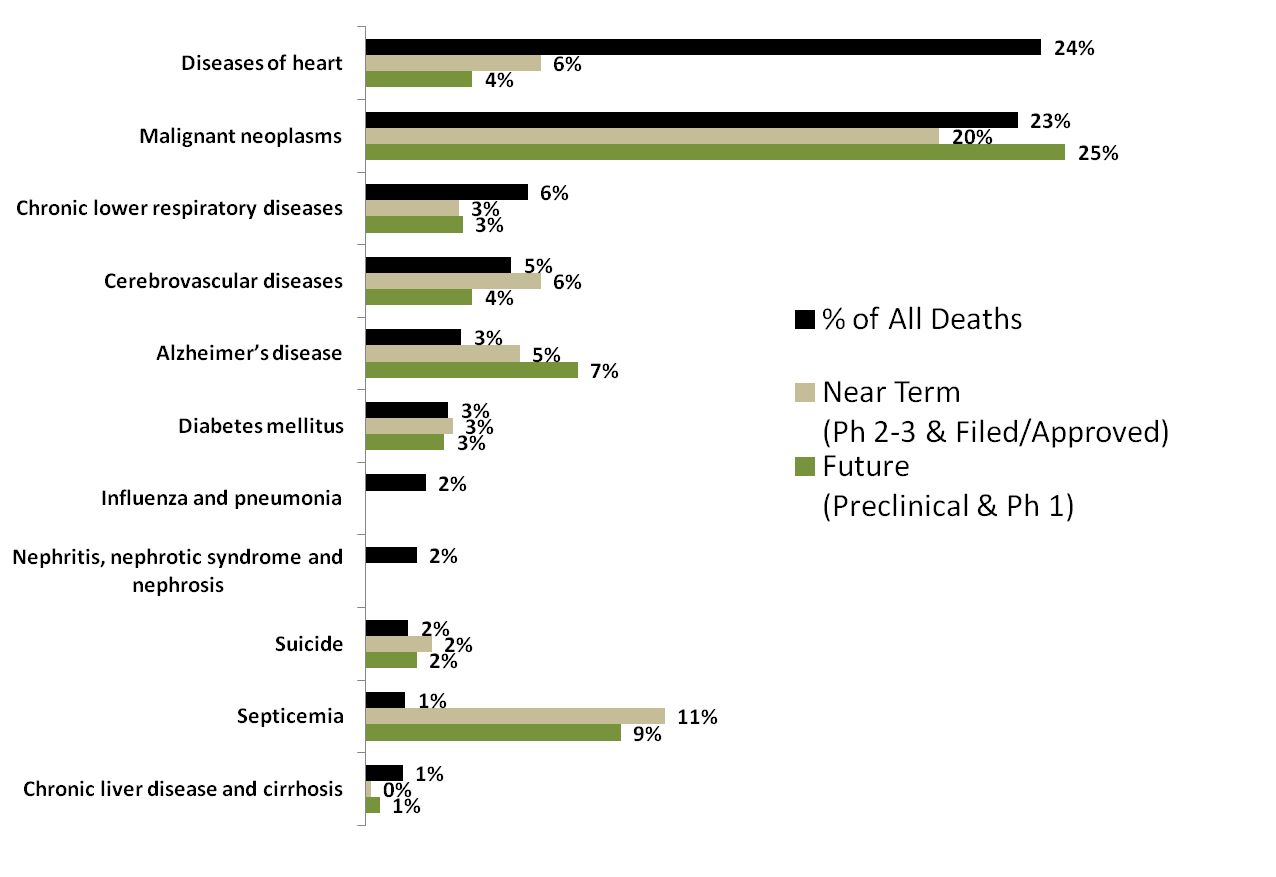
Fifteen demonstration projects have been funded by the NIH to demonstrate hypothesized correlations between the microbiome and human health and disease”:
- Evaluation of the cutaneous microbiome in psoriasis
- The Vaginal Microbiome: Disease, Genetics and the Environment
- Diet, Genetic Factors, and the Gut Microbiome in Crohn’s Disease
- The Role of the Gut Microbiota in Ulcerative Colitis
- Urethral Microbiome of Adolescent Males
- The Thrifty Microbiome: The Role of the Gut Microbiota in Obesity in the Amish
- Metagenomic Analysis of the Structure and Function of the Human Gut Microbiota in Crohn’s Disease
- Effect of Crohn’s Disease Risk Alleles on Enteric Microbiota
- Metagenomic study of the human skin microbiome associated with acne
- Foregut microbiome in development of esophageal adenocarcinoma
- The Microbial Ecology of Bacterial Vaginosis: A Fine Scale Resolution Metagenomic
- Skin Microbiome in Disease States: Atopic Dermatitis and Immunodeficiency
- The human virome in children and its relationship to febrile illness
- The Neonatal Microbiome and Necrotizing Enterocolitis
- The Human Microbiome in Pediatric Abdominal Pain and Intestinal Inflammation
Does Bacteria Therapy Work?
Patients with symptoms of Clostridium difficile (Cdiff) infection have been successfully treated by essentially replacing their current population of fecal bacteria with fecal bacteria from well people. These fecal transplants (examples here, here, and here) are typically very effective and well tolerated. It seems only reasonable to expect similar positive results for other health conditions being improved by microbiome adjustment.
What is Next?
What is likely to happen is the continued clinical studies of adjusting patients’ microbiomes in the hope of improving health. As these studies continue and positive evidence accumulates, the pharmaceutical industry will develop commercial products to meet the clinical needs that the new science can satisfy. What I think will be important for this area is how intellectual property protection is granted given that the bacteria are essentially prior art.
Information Sources
Here are links to some additional sources of information related to the microbiome and health:
- The Human Microbiome Project Consortium,”Structure, function and diversity of the healthy human microbiome”, June 14, 2012, Vol. 486, Nature, 207-14.
- “Me, myself, us”, The Economist, Aug 18th 2012.
- Special collection of articles in Nature on microbiome
- “Special Issue on the Gut Microbiota”, Science, 6 June 2012.
- J. L. Sonnenburg, M. A. Fischbach, Community health care: Therapeutic opportunities in the human microbiome. Sci. Transl. Med. 3, 78ps12 (2011)
- MetaHIT website